Product: LumiraDx SARS-CoV-2 Ag Test
Rapid results in minutes at the point of care
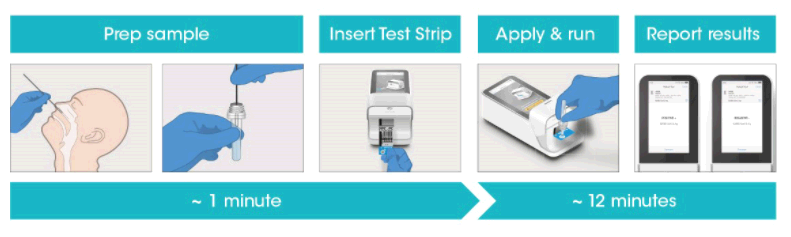
The LumiraDx SARS-CoV-2 Ag Test is a microfluidic immunofluorescence assay for direct and qualitative detection of nucleocapsid proteins in nasal swab specimens from patients suspected of COVID-19. Used with the LumiraDx Instrument the Test delivers rapid results at the point-of-care.
Test Benefits
The use of a LumiraDx SARS-CoV-2 Ag Test on the LumiraDx Instruments will enable the physician to verify infection quickly, begin proper treatment and to initiate isolation precautions helping prevent further spread of infection.
- Easy to implement in point-of-care settings
- Clinical performance
97.6% positive percent agreement
96.6% negative percent agreement - Analytical performance with a limit of detection of 32 TCID50/mL
- RT-PCR comparable results within 12 days of onset of symptoms
Link to LumiraDx website